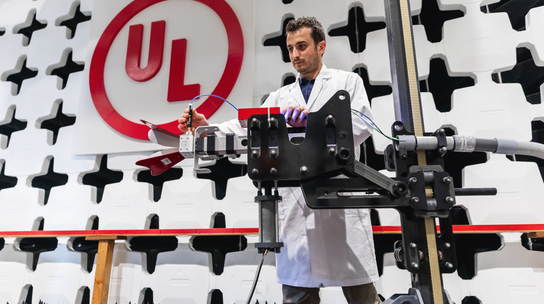
JOB DESCRIPTION
Our CMIT (Consumer, Medical & Information Technologies) has a fantastic opportunity available for an Intern to join our Medical Devices team in Basingstoke for 1 year. Our aim is to be able to offer a Full-Time permanent job role upon completion of the Internship. As an Intern, you'll provide vital support to both Laboratory Engineers who are testing a range of medical devices in the lab, and to our Project Engineers who work closely with the customer to ensure the successful delivery of their projects.
This is a great opportunity for you to take your 1 st step on the career ladder in the world of safety testing and certification. Not only will you gain hands-on, real world testing experience in a laboratory, but you'll also gain experience of working directly with high profile customers across Europe.
RESPONSIBILITIES
Project Handling Responsibilities
- Work with our Project Engineers to determine project scope, develop a preliminary plan of investigation, and determine project specifications such as cost, time, and sample requirements
- Communicate with clients to discuss technical issues, explain UL procedures and requirements, convey project cost, and negotiate completion date and sample requirements.
- Provides technical assistance to clients in reference to product inspection and follow-up services.
- Establishes appropriate test programs by reviewing files and manufacturer's information, examining samples, and applying UL requirements.
- Determine areas in which the product is not in compliance with UL requirements or of any changes in project scope or specifications and notifies client.
- Builds and/or adapts setup and test equipment to new situations, based on data sheets and instructions from engineering department personnel
- Operates designated laboratory equipment
- Undertake testing on a wide range of products requiring certification
- General laboratory related maintenance / upkeep
- Accurately documenting test findings, which appropriately detail all aspects of testing performed
- To update the company, work systems, with details of testing and overall job status, for each item of work for which the individual is responsible
Testing Responsibilities
- Builds and/or adapts setup and test equipment to new situations, based on data sheets and instructions from engineering department personnel
- Operates designated laboratory equipment
- Undertake testing on a wide range of products requiring certification
- General laboratory related maintenance / upkeep
- Accurately documenting test findings, which appropriately detail all aspects of testing performed
- To update the company, work systems, with details of testing and overall job status, for each item of work for which the individual is responsible
QUALIFICATIONS
- A University Degree in Electrical or Electronics Engineering is preferred, but a HND in Electrical or Electronics Engineering will also be considered
- Knowledge of electronics is key, with the ability to understand electrical circuits
- A passion for solving problems and resolving technical issues
- An interest in project handling / project management would be highly beneficial
- Customer service experience is preferred, as you will at times be directly communicating with our customers
- Ability to work independently whilst also being able to work in a team environment
#LI-AV1
ABOUT US
A global leader in applied safety science, UL Solutions (NYSE: ULS) transforms safety, security and sustainability challenges into opportunities for customers in more than 110 countries. UL Solutions delivers testing, inspection and certification services, together with software products and advisory offerings, that support our customers' product innovation and business growth. The UL Mark serves as a recognized symbol of trust in our customers' products and reflects an unwavering commitment to advancing our safety mission. We help our customers innovate, launch new products and services, navigate global markets and complex supply chains, and grow sustainably and responsibly into the future. Our science is your advantage.
ABOUT THE TEAM
Every product needs to be safe. But for medical devices, it's absolutely essential. For manufacturers, getting medical devices approved can be incredibly complex. That's where our team of experts come in. Together, we help improve medical outcomes. We test, advise and use our world-class knowledge to help manufacturers navigate changing standards and regulations, so they can provide people around the world with innovative products that save and improve lives. Whether it's equipment to help someone walk again or an incubator for a premature baby, our experts are behind them all, helping to ensure their safety. Join our team, connect with global experts and help shape the next generation of medical technology.

What We Do
A global leader in applied safety science, UL Solutions transforms safety, security and sustainability challenges into opportunities for customers in more than 100 countries. UL Solutions delivers testing, inspection and certification services, together with software products and advisory offerings, that support our customers’ product innovation and business growth. The UL Certification Marks serve as a recognized symbol of trust in our customers’ products and reflect an unwavering commitment to advancing our safety mission. We help our customers innovate, launch new products and services, navigate global markets and complex supply chains, and grow sustainably and responsibly into the future. Our science is your advantage.
Why Work With Us
Science is in our DNA; we are endlessly curious and passionate about seeking and speaking the truth. We take delight in knowing that our work makes a meaningful contribution to society, and we are proud that our culture is centered on integrity, collaboration, inclusion and excellence.
Gallery










UL Solutions Teams
UL Solutions Offices
Hybrid Workspace
Employees engage in a combination of remote and on-site work.
Depending on the role we offer hybrid or remote opportunities.